Chinese expert consensus on refined diagnosis, treatment and management of advanced primary liver cancer: 2023 edition
Published 23 July, 2024
Hepatocellular carcinoma (HCC), commonly known as primary liver cancer, is a major cause of malignant tumors and cancer-related deaths worldwide. In China, the condition accounts for approximately 85% of all cancer cases. While several guidelines have been used to diagnose and treat liver cancer, they only provide a broad definition for classifying advanced liver cancer with an emphasis on a singular approach. Notably, the consideration for personalized treatment options has largely been lacking.
To that end, a team of experts led by Fusheng Wang from The Fifth Medical Center of PLA General Hospital in Beijing adopted the Delphi method and established a comprehensive and practical expert consensus specifically for China. The classification criteria were refined for Chinese patients with HCC, and a corresponding set of optimal treatment regimen recommendations was developed accordingly.
“These recommendations took into account various factors, including tumor characteristics, vascular tumor thrombus grade, distant metastasis, liver function status, portal hypertension, and the hepatitis B virus replication status of patients with primary HCC, along with treatment prognosis,” explains Wang.
The findings and recommendations provide detailed, scientific, and reasonable individualized diagnosis and treatment strategies for clinicians. The consensus, published in the KeAi journal Liver Research, is summarized as follows:
- Classification
Recommendation 1: Child-Pugh class B7 is recommended as the primary threshold for precisely classifying the liver function status of advanced liver cancer (with a 95% consensus agreement among experts).
Recommendation 2: Cheng's classification is recommended as the reference criterion for the precise classification of advanced liver cancer with portal vein tumor thrombus (PVTT) (with a 100% consensus agreement among experts).
Recommendation 3: The definition of liver cancer oligometastasis (≤5 metastatic/recurrent lesions and ≤2 involved organs) is recommended as the reference criteria for characterizing extrahepatic metastatic lesions and providing treatment decision guidelines for the precise classification of advanced liver cancer (with a 95% consensus agreement among experts).
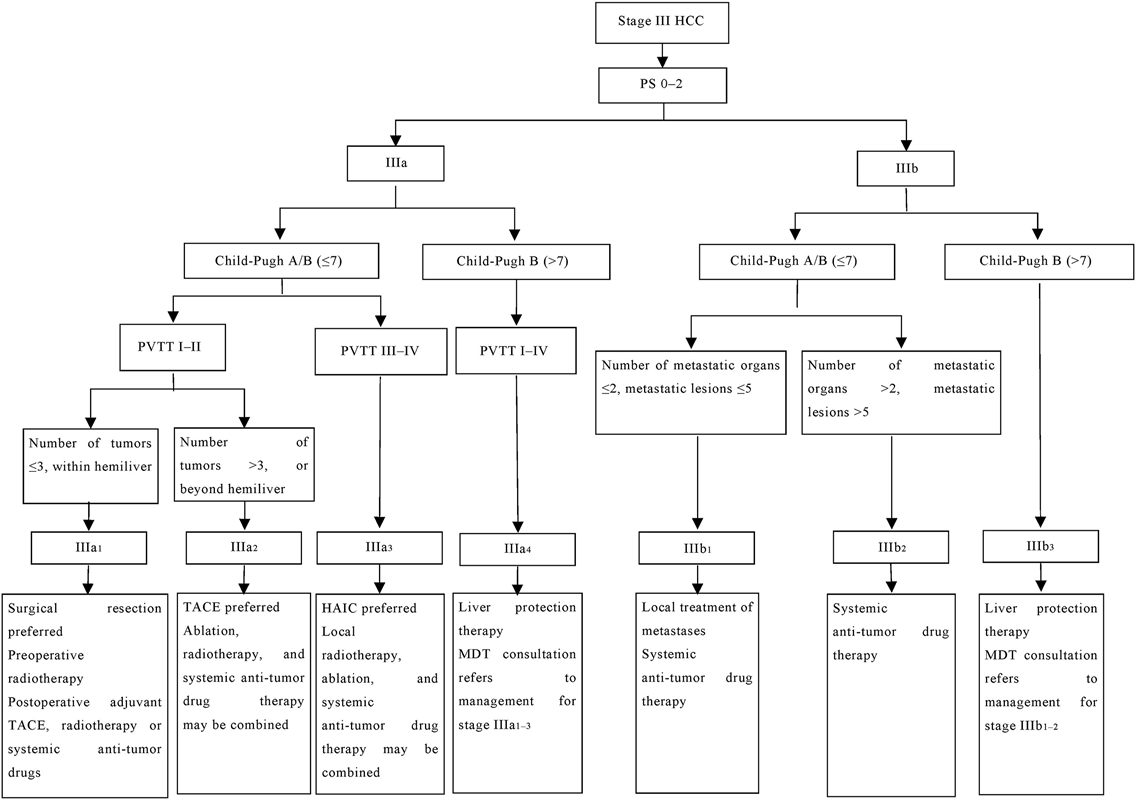
Recommendation 4: Based on the above evidence, the recommended classification criteria for advanced liver cancer were as follows (see Fig. 1 for the specific staging route):
- Stage IIIa1: PS 0–2, Child-Pugh class A/B (≤7 points), PVTT (type III), tumors confined to the hemiliver and ≤3 in number, with sufficient residual liver volume and function, and without evidence of extrahepatic metastasis.
- Stage IIIa2: PS 0–2, Child-Pugh class A/B (≤7 points), PVTT (type I–II), >3 tumors, or extension beyond one hemiliver, and without evidence of extrahepatic metastasis.
- Stage IIIa3: PS 0–2, Child-Pugh class A/B (≤7 points), PVTT (type III–IV)/vena cava tumor thrombus, and without evidence of extrahepatic metastasis.
- Stage IIIa4: PS 0–2, Child-Pugh class B (>7 points), PVTT (type I–IV) and without evidence of extrahepatic metastasis, irrespective of tumor condition.
- Stage IIIb1: PS 0–2, Child-Pugh class A/B (≤7 points), and the presence of oligometastasis in extrahepatic lesions, regardless of tumor conditions and vascular tumor thrombus.
- Stage IIIb2: PS 0–2, Child-Pugh class A/B (≤7 points), and presence of extrahepatic lesion metastasis extending beyond the definition of oligometastasis, regardless of tumor conditions and presence of vascular tumor thrombus.
- Stage IIIb3: PS 0–2, Child-Pugh class B (>7 points), with extrahepatic lesion metastasis, regardless of tumor conditions and presence of vascular tumor thrombus.
- Classification and management of patients with stage IIIa
Recommendation 5: Patients with the following criteria are recommended for surgical resection as the preferred treatment option: Child-Pugh class A or B (≤7 points), tumors confined to the hemiliver and ≤3 in number, resectable primary lesions, adequate residual liver volume and function, PVTT type I–II, and PS 0–1 (with a 91% consensus agreement among experts).
Recommendation 6: Patients with PVTT type I–II are recommended to undergo preoperative three-dimensional conformal radiotherapy or postoperative adjuvant TACE, radiotherapy, or systemic anti-tumor drugs. This process is aimed at delaying or reducing postoperative recurrence (with a 91% consensus agreement among experts).
Recommendation 7: Patients in Child-Pugh class A or B (≤7 points) with unresectable primary lesion, PVTT type I–II, >3 tumors, tumors extending beyond a single hemiliver, and PS 0–1 are recommended to receive local TACE first, combined with ablation, radiotherapy, and systemic anti-tumor drug therapy (with a 91% consensus agreement among experts).
Recommendation 8: For patients who poorly responded to TACE alone, TACE combined with systemic anti-tumor drug therapy should be initiated earlier (with a 95% consensus agreement among experts).
Recommendation 9: Patients in Child-Pugh class A or B (≤7 points) with unresectable primary lesions, PVTT type III–IV, well-developed hilar collateral circulation, and PS 0–1 may be treated with HAIC (with an 86% consensus agreement among experts). The combination drug therapy and management regimen should follow the guidelines outlined for the systemic treatment of patients in stage IIIb2.
Recommendation 10: Patients in Child-Pugh class A or B (≤7 points) with liver cancer and unresectable primary lesion, PS 0–1, and PVTT III–IV may consider radiotherapy for the primary lesion and PVTT (with a 91% consensus agreement among experts). For the combined drug therapy and management regimen, it is advisable to refer to the guidelines provided for the systemic treatment of patients in stage IIIb2.
Recommendation 11: For patients with advanced liver cancer defined as Child-Pugh class B (>7 points), the restoration of liver function to Child-Pugh B7 should be prioritized before initiating the anti-tumor treatment. During this phase, incorporating Icaritin treatment can be considered, or the treatment approach can be determined following consultation with an MDT, including hepatologists (with a 95% consensus agreement among experts).
- Classification and management of patients in stage IIIb
Recommendation 12: For patients with Child-Pugh class A or B (≤7 points) and extrahepatic oligometastasis, active local treatment is recommended (with an 86% consensus agreement among experts).
Recommendation 13: Patients categorized under Child-Pugh class A or B (≤7 points) with multiple extrahepatic metastases are best suited for systemic therapy. Decisions regarding local treatment of these metastases should be made following an MDT discussion (with a 100% consensus agreement among experts).
Recommendation 14: For patients with Child-Pugh class B (>7 points) with extrahepatic metastasis, restoring liver function to Child-Pugh B7 before the anti-tumor treatment is recommended. During the period, systemic anti-tumor drugs and other treatments can be selected after MDT discussion (with a 100% consensus agreement among experts).
- Antiviral management for patients with virus-associated advanced liver cancer
Recommendation 15: Patients with HBV/HCV-related liver cancer and positive HBsAg/HCV RNA should promptly begin antiviral therapy (with a 100% consensus agreement among experts).
Recommendation 16: Patients with HBV and HCV-related liver cancer receiving anti-tumor therapy are at risk of viral reactivation. HBV DNA, HCV RNA, and liver function status should be closely monitored during treatment and antiviral treatment strategies adjusted in time (with a 100% consensus agreement among experts).
- Systemic treatment and management of advanced liver cancer complicated with portal hypertension
Recommendation 17: Both liver cancer treatment and portal hypertension management should be established for patients with portal hypertension and liver cancer. The treatment regimen containing bevacizumab should be carefully considered in choosing a systemic treatment plan for patients with severe portal hypertension, particularly those with severe esophagogastric varices with red color signs. Other targeted drugs can be considered as alternatives, with close monitoring by doctors (with a 95% consensus agreement among experts).
Contact author name, affiliation, email address:
Yinying Lu, Comprehensive Liver Cancer Center, The Fifth Medical Center of PLA General Hospital, Beijing, China.
E-mail address: luyinying1973@163.com.
Funder:
This work was funded by the National Natural Science Foundation of China (No. 82272956).
Conflict of interest:
The authors declare that they have no conflict of interest. This article is based on a study first published in Zhonghua Gan Zang Bing Za Zhi. Translated and reprinted by permission of Chinese Medical Association.
Please paste here the Declaration of Competing Interest statement from your published article.
See the article:
Liu, X., et al., Chinese expert consensus on refined diagnosis, treatment, and management of advanced primary liver cancer (2023 edition), Liver Res, Volume 8, Issue 2, 2024, Pages 61-71, https://doi.org/10.1016/j.livres.2024.05.001.