The miracle of taVNS: a new non-invasive therapy offering hope for multiple diseases
已发布 28 六月, 2024
Vagus nerve stimulation (VNS) has been recognized by the U.S. Food and Drug Administration (FDA) as an effective alternative therapy for a wide range of diseases. However, its widespread application is hampered by various issues, including high surgical risks and the long-term presence of a foreign body within the patient.
Transcutaneous auricular vagus nerve stimulation (taVNS) is a non-invasive, low-cost method that combines principles from vagus nerve stimulation and traditional Chinese medicine's auricular acupoint theory. It aims to replace surgically implanted vagus nerve stimulation in the treatment of disease. Unlike traditional acupuncture, taVNS can be self-administered wherever possible, making it highly accessible and non-invasive.
Now, a team of researchers from China detailed the foundational research progress and clinical applications of taVNS in a commentary published in the World Journal of Acupuncture-Moxibustion.
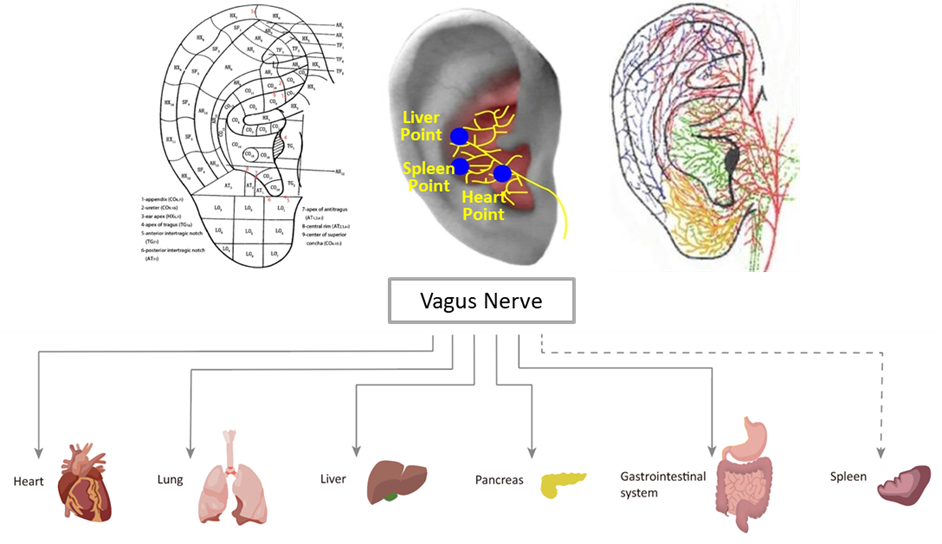
“Compared with other auricular points, taVNS can regulate internal organ function by stimulating specific points on the ear. Initially used as an alternative therapy for epilepsy, taVNS has been extended to treat central nervous system disorders, cardiovascular diseases, digestive system diseases, and endocrine system disorders,” explains the study's senior author, Shao-yuan Li, an associate researcher at the Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences.
TaVNS has shown significant effects in various body systems. It has effective in reducing depression scores and treating conditions such as migraines, anxiety, cognitive disorders, Parkinson’s disease, and cognitive decline. Additionally, it has demonstrated benefits for the cardiovascular system by lowering blood pressure and heart rate, treating arrhythmias and myocardial infarction, and alleviating cardiac insufficiency and heart failure. Furthermore, it has proven effective for treating functional gastrointestinal diseases, glucose-lipid metabolism disorders, endotoxemia, rheumatoid arthritis, systemic lupus erythematosus, as well as comorbid chronic diseases with depression.
“In recent years, clinical research on taVNS has provided substantial evidence for its application, facilitating its transition from the laboratory to medical practice and expanding into areas such as sleep disorders, cognitive impairments, and neurodegenerative diseases,” adds Li. “Since 2000, there has been a steady increase in taVNS-related publications and achievements, with a significant contribution from China.”
According to Pei-jing Rong, lead investigator of the study, this is an excellent treatment in neural regulation therapy. “Technological advancements have made taVNS devices more accessible for patients. To anticipate taVNS being applied in more areas and offer personalized and effective treatment options in the whole world, we are promoting the progress of International Organization for Standardization (ISO) development”
Contact author details:
Shao-yuan LI, Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing 100700, China, 704488328@qq.com.
Pei-jing RONG, Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing 100700, China, drrongpj@163.com.
Funder:
Supported by Young Elite Scientists Sponsorship Program by CAST: 2021-2023ZGZJXH-QNRC003, Beijing TCM Science and Technology Development Fund Project: BJZYON-2023-05 and Fundamental Research Funds for the Central Public Welfare Research Institutes: ZZ202219002, ZZ-YQ2023006.
Conflict of interest:
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
See the article:
Yu-zheng-heng Zhang, et al., Transcutaneous auricular vagus nerve stimulation: clinical applications and mechanisms, World Journal of Acupuncture-Moxibustion, 2024, 34(02), Pages 174-175. https://doi.org/10.1016/j.wjam.2024.03.007.